Research may reveal how rabies induces frenzied behavior
October 9, 2017
Meghan Murphy
907-474-7541

Scientists may finally understand how the rabies virus can drastically change its host’s behavior to help spread the disease, which kills about 59,000 people annually.
A new study published in the journal Scientific Reports shows how a small piece of the rabies virus can bind to and inhibit certain receptors in the brain that play a crucial role in regulating the behavior of mammals. This interferes with communication in the brain and induces frenzied behaviors that favor the transmission of the virus.
Dr. Karsten Hueffer, lead author and a professor of veterinary microbiology at the University of Alaska Fairbanks, said he hopes the findings will help scientists better understand and treat the infectious viral disease.
“Many infectious agents change behavior in their host, but we do not understand how they do this,” he said. “Our study provides, for the first time, a detailed molecular mechanism for how an infectious agent induces specific behaviors.”
Hueffer said that although vaccinations for rabies were first developed in the mid-19th century, scientists have struggled to explain how the virus can induce aggressive behavior in animals like dogs, which contribute up to 99 percent of all rabies transmissions to humans, according to World Health Organization estimates.
“The rabies virus only has five genes and very little information,” he said. “Dogs have more than 20,000 genes with sophisticated immune and central nervous systems. Yet this virus can reprogram a dog’s behavior so it loses fear, becomes aggressive and bites, which allows the virus to spread through the dog’s saliva.”
Hueffer said these behavioral changes are well known and cemented into the American narrative. In the novel "Old Yeller," Travis must put down his beloved dog after it is bitten and infected by a rabid wolf. In “To Kill a Mockingbird,” Atticus Finch is called to shoot a rabid dog.
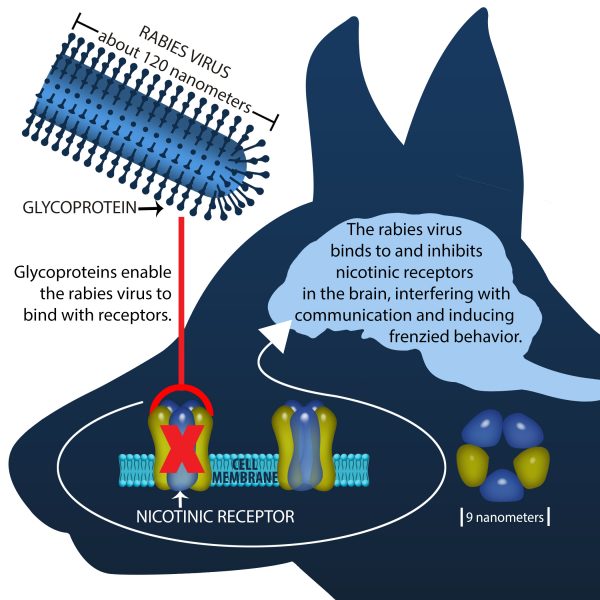
“Yet the behavior is easier to study than the virus itself,” said Hueffer. He said studying rabies in the brain is difficult because the rabies virus doesn’t physically alter the brain in very telling ways. Usually, there is brain inflammation, but scientists must sample the brain and specifically test for rabies virus to confirm infection.
Prior research in the 1980s and 1990s focused on how the rabies virus binds to and interacts with specific muscle receptors that receive signals from nerves to control muscle contraction. The research revealed that a molecule called a glycoprotein on the surface of the rabies virus can bind to nicotinic acetylcholine receptors in the muscles. The virus then enters and hijacks muscle and nerve cells where it replicates and travels up the nerves to infect the brain and other tissues.
Other research found a string of amino acids within the rabies glycoprotein that is almost identical to an amino acid sequence found in snake venom that inhibits nicotinic acetylcholine receptors.
Hueffer said he and co-author Marvin Schulte realized a connection. Schulte, an expert on nicotine receptors, is a former UAF professor now at the University of the Sciences in Philadelphia.
“Dr. Schulte and I put two and two together,” Hueffer said. “We knew that nicotinic acetylcholine receptors, which bind to the virus in muscles, are also found in the brain, and we presumed that virus could also bind to such receptors. If snake venom has a similar structure to parts of the virus, and inhibits these receptors, we thought maybe the virus could also inhibit these receptors in the brain. Furthermore, we thought that this interaction could influence behavior.”
Hueffer then teamed with another co-author, Michael Harris, to develop experiments to demonstrate whether the rabies virus glycoprotein alters behavior in animals. Harris, also formerly of UAF, is now a professor at California State University Long Beach.
“The viruses collect in the spaces between brain cells during the early stages of infection,” Harris said. “These spaces are where brain cells communicate. We thought that if viruses could bind to receptors in these spaces and change how brain cells normally communicate, the virus could change behavior of the infected animal.”
This change of behavior could work to the advantage of the virus, changing the behavior of infected animals to increase the chances that infection will spread to other animals.
In one of the experiments, Hueffer and his colleagues injected a small piece of the rabies virus glycoprotein into the brains of mice.
“When we injected this small piece of the virus glycoprotein into the brain of mice, the mice started running around much more than mice that got a control injection,” he said. “Such a behavior can be seen in rabies infected animals as well.”
Hueffer said that this is the first experimental evidence showing a molecular mechanism inducing a specific behavioral change in a host that favors a disease’s transmission.
While rabies is now rare in the United States and largely preventable through vaccinations, there is no known cure once symptoms occur. This viral disease is still devastating poor, rural regions mostly in Africa and Asia that lack the means to vaccinate dogs or provide treatment if infection is suspected.
ADDITIONAL CONTACTS: Karsten Hueffer, khueffer@alaska.edu
Paper online: http://rdcu.be/wAg4


